Catalytic Converter
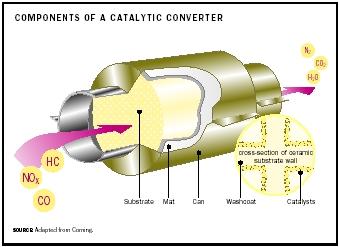
The catalytic converter in an automobile is an expanded section of exhaust pipe occurring upstream of the muffler in which pollutants generated in the engine are converted to normal atmospheric gases. It is an essential element in the emissions control system of modern automobiles. This technology was introduced in the United States in the late 1970s and became legally required by the early 1980s because of more stringent exhaust emission control standards. Early catalyst systems, as applied to vehicles with carburetors, attempted to oxidize carbon monoxide (CO) and unburned hydrocarbons (HC) to carbon dioxide (CO 2 ) and water vapor, using air added by means of an air pump or rapidly actuating valve system. Although constructed from a high-surface-area alumina substrate with a noble metal (usually platinum) on the surface, their effectiveness was limited by extreme conditions of service. These problems include high temperatures (greater than 1,000°C) exacerbated by a large and variable "engine-out" pollutant load and constant vibration from roadway and engine sources.
The replacement of carburetors with computer-controlled, port fuel injection and precise air/fuel ratio control based on exhaust oxygen sensing has allowed catalytic converters to operate with close to 100 percent efficiency and better longevity, often exceeding 100,000 miles. The addition of a ceria wash coat in the form of a thin layer of porous cerium oxide and
SEE ALSO G REENHOUSE G ASES ; L EAD ; O ZONE ; V EHICULAR P OLLUTION .
Internet Resource
Kovark, William, and Hermes, Matthew E. "The Role of the Catalytic Converter in Smog Reduction." Available from http://chemcases.com/converter .
Donald Stedman
Comment about this article, ask questions, or add new information about this topic: